by Praveen Raju , Renuka Abraham and Swarna Jain
I. INTRODUCTION
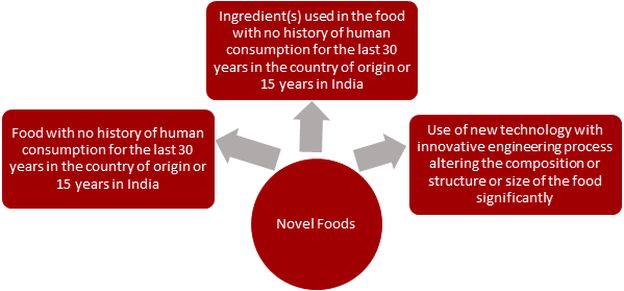
Increased connectivity around the world has started a new wave in the market for novel foods. Novel foods such as genetically modified foods and food products, cell-based meat, eggs, and dairy alternatives, as well as plant-based and fermentation-derived proteins, have entered the Indian market. Therefore, it becomes important to understand the regulatory framework put in place for novel foods. Under the Food Safety and Standards (Health Supplements, Nutraceuticals, Food for Special Dietary Use, Food for Special Medical Purposes, Functional Food, and Novel Food) Regulations, 2016 (“2016 Regulations”), novel foods are defined as:
In the Indian plant-based food market, several players like Good Dot and One Good feature a wide range of novel food products including soy milk, coconut milk, almond cheese, and plant-based meat alternatives, among others. In addition, an Indian start-up, Clear Meat, has successfully developed and tested its first cultivated chicken mince, with plans to launch its market-ready product by 2023. Myoworks also aims to manufacture ingredients and scaffolds for the cultivated-meat industry and has received INR 50 lakhs from the Department of Biotechnology, India to demonstrate a preliminary proof of concept.
Plant-based alternatives are gaining popularity worldwide with start-ups like Zero Egg and Vly Foods offering a multitude of products. Besides, Eat Just, a Californian company, made history in December 2020, when its cultivated chicken became the first cultured meat product to receive regulatory approval in Singapore.

II. REGULATIONS GOVERNING NOVEL FOODS IN INDIA
The Food Safety and Standards Authority of India (“FSSAI“), on 11 September 2017, notified the Food Safety and Standards (Approval of Non-Specified Food and Food Ingredients) Regulations, 2017 (“2017 Regulations“). Under the 2017 Regulations, prior approval is required for certain articles of food or food ingredients, before its introduction in the market.
In addition, the 2016 Regulations govern eight categories of foods including novel foods. The regulations provide a list of ingredients and additives that are allowed to be used in specified food categories. The Food Business Operators (“FBO“) intending to manufacture, import or sell the specified foods are required to adhere strictly to these regulations.
Although in March 2022, the Food Safety and Standards (Health Supplements, Nutraceuticals, Food for Special Dietary Use, Food for Special Medical Purpose, and Prebiotic and Probiotic Food) Regulations, 2022 (“2022 Regulations“) was enacted, superseding the 2016 Regulations, the 2022 Regulations specifically excludes novel foods from the applicability of the regulations and therefore the 2016 Regulations and 2017 Regulations continue to govern novel foods.

Recently, in 2022, the FSSAI notified the Food Safety and Standards (Vegan Foods) Regulations, 2022 (“Vegan Regulations“) which govern plant-based food products which are novel foods. The Vegan Regulations stipulate the definition of vegan, requirements as a part of the prior-approval process and labelling declarations, among others.
III. License for Novel Food Manufacturers
Novel food businesses are required to obtain a central license under the Food Safety and Standards (Licensing and Registration of Food Business) Regulations, 2011 (“2011 Regulations“) from the licensing authorities, whereas petty FBOs involved in manufacturing of novel foods are only required to register themselves under regulations. In addition to the specific procedures for license application, FBOs are required to comply with hygienic and safety practices. The 2011 Regulations also necessitate all renewals and changes in the business to be appropriately communicated and approved by the licensing authority. A novel food manufacturer or importer needs prior approval from FSSAI as per the 2017 Regulations before applying for a central licence.

** Click here to read the full-text **








