Quotes from The Grocer, March 22, 2024
By Ian Quinn
Efforts to expedite the regulatory approval process may have implications for lab-cultured meat, innovative food products, and cannabidiol (CBD). However, there is a perspective that suggests such measures might not be implemented in time to be effective.
The Vote Leave campaign did not significantly highlight the potential for a more rapid introduction of CBD products, insect-based proteins, and cultured meats to the marketplace as an advantage of leaving the EU.
The United Kingdom’s newfound autonomy in crafting regulations for numerous cutting-edge technologies, which were once hindered by the well-known bureaucratic hurdles of Brussels, is now highlighted as one of the potential advantages derived from the referendum outcome.

Food enterprises continue to encounter a significant delay, averaging two and a half years, for obtaining necessary approvals. This bottleneck affects numerous products that are part of an industry with the potential to generate billions in revenue, all of which are currently awaiting their turn.
Could the recent initiatives suggested by the Food Standards Agency transform the United Kingdom into a global hub for food technology innovation, or is it possible that we are trailing in progress?
The proposed comprehensive reforms encompass a wide range of industries within the scope of controlled food substances, which consist of food enhancers, substances added to animal feed, and taste-enhancing agents.
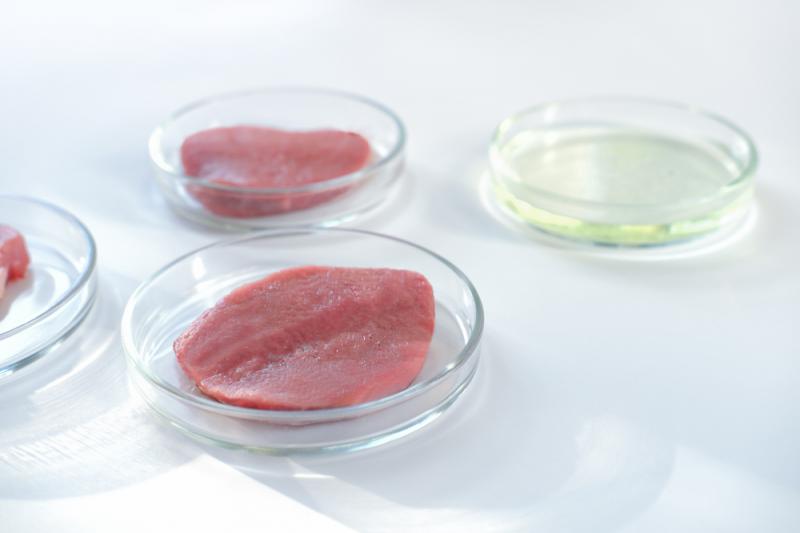
Several industries are at the forefront of current discussions, notably genetically modified (GM) foods, innovative food sources like edible insects, the expanding market of CBD-infused products, and the emergent field of laboratory-cultivated meat. These areas are shaping the conversation around food technology and its future direction.

The Food Standards Agency is urging Members of Parliament to eliminate regulations that are causing a backlog of 470 applications. This change would remove the necessity for Members of Parliament to enact a statutory instrument during the approval process, and instead, establish a publicly accessible, official list of products authorized by the FSA with the consent of a minister. Additionally, it would abolish the need for decennial re-authorization of products such as smoke flavorings, animal feed additives, and genetically modified foods.
** To access the complete text, please click here **